Clinical trials
Están publicados los resultados de estudios clínicos
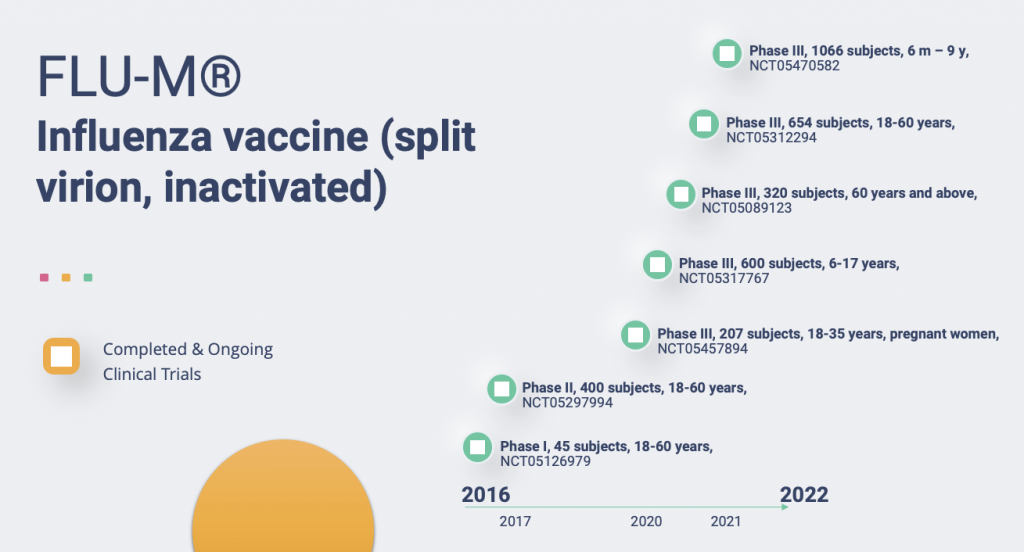
- The Objectives of This Study Are Study of the Reactogenicity, Safety and Immunogenicity of Flu-M Inactivated Split Influenza Vaccine in Volunteers Aged 18-60 Years
- Trial of the Reactogenicity, Safety and Immunogenicity of the Flu-M Vaccine Manufactured by FSUE SPbSRIVS FMBA
- Tolerability, Safety and Immunogenicity Trial of the Flu-M® Inactivated Vaccine in Volunteers Aged 18 to 60 Years
- The Objectives of This Study Are Study the Immunogenicity and Safety of the Flu-M [Inactivated Split Influenza Vaccine], vs. the Ultrix® Inactivated Split Influenza Vaccine, in Volunteers Who Are Over 60 Years Old
- Trial of Tolerability, Reactogenicity, Safety and Immunogenicity of Flu-M [Inactivated Split Influenza Vaccine] in Pregnant Women
- Trial of Tolerability, Safety and Immunogenicity of the Flu-M Vaccine in Children Between 6 Months and 9 Years Old
- The Objectives of This Study Are Comparative Assessment of the Tolerability, Safety and Immunogenicity of the Flu-M Vaccine vs. the Ultrix® Vaccine by Single Vaccination of Children Aged 6 to 17 Years.
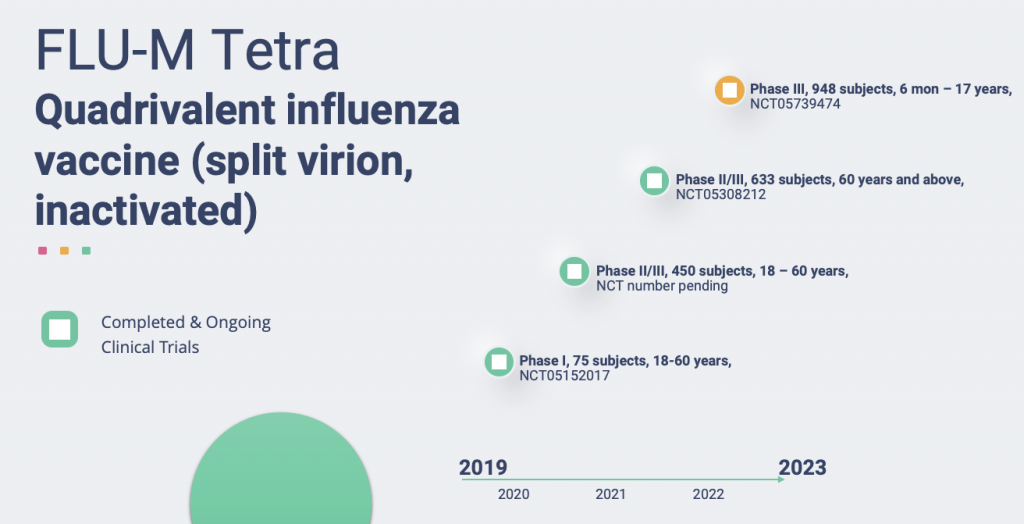
- The Objectives of This Study Are Study the Safety, Reactogenicity and Obtain Preliminary Data on the Immunogenicity of Flu-M Quadro, 4-valent Inactivated Split Influenza Vaccine, in Healthy Volunteers
- Tolerability, Safety and Immunogenicity Trial of the FLU-M® Tetra
- Tolerability, Safety and Immunogenicity Trial of the Flu-M Tetra Vaccine in Children